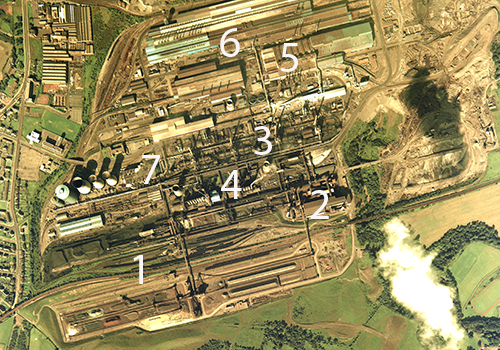
Steelworks
Steel is vital to the national economy and to everyday life; it is used in construction, transport infrastructure and in the manufacture of goods and foods. Although steel is produced on extensive industrial sites, where the co-location of several associated processes can make them appear bewildering to interpret, they are generally laid out with a production flow in mind.
This feature outlines each of the processes involved in steelmaking and identifies them on aerial imagery of the former Ravenscraig steelworks, Lanarkshire, Scotland.
|
1. RAW MATERIALS
The iron and steel-making process requires iron ore, coal and limestone. These raw materials are transported to the steel plant in bulk by ship and rail and stored in stockyards adjacent to the plant. Reclaimers distribute the ore into beds, where it is mixed to produce the most suitable blend.
From the stockyards, coal is moved by conveyor to the coke ovens, while iron ore is mixed with coke and sent to the sinter plant.
|
 |
|
2. SINTERING
In the sinter plant, iron ore and coke particles are fed through an oven, where the mixture is cooked to drive off impurities such as sulphur. The result is a solid clinker, known as sinter, which aids the efficient smelting of iron ore. The sinter plant is connected to the stockyard by a series of conveyors and is equipped with electrostatic precipitators to remove ash particles from flue gases before they reach the chimney stack.
|
 |
|
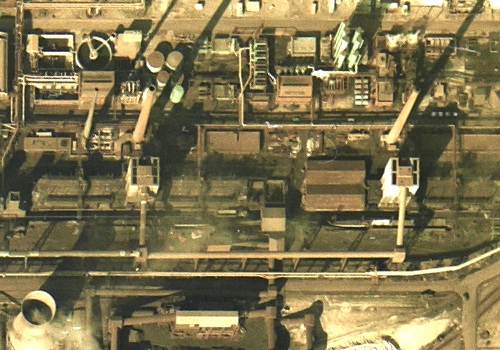
3. COKE PRODUCTION
Coke is produced by heating coal in an oven, to drive out oil and tar before it is used in the blast furnace. A quenching car moves along rails parallel to a battery of coke ovens and transports hot coke, rammed into the car from the ovens, to the quenching tower. Here it is extinguished by water, and then dumped onto the coke-drying wharf. From there, it is graded in a screening tower before being moved to the blast furnace.
A plume of steam on aerial imagery of a coke battery indicates an active quenching tower.
|
 |
|
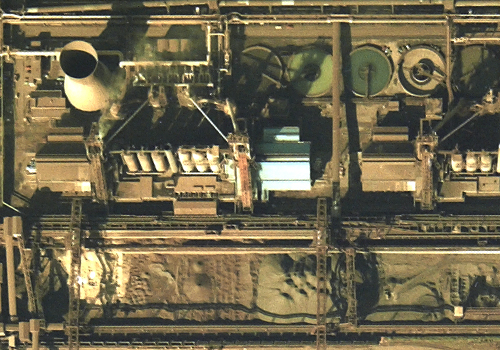
4. IRON PRODUCTION
Iron ore sinter, coke and limestone are tipped into the blast furnace, where their combustion is aided by very hot air blown in from an adjacent row of stoves. The iron ore reduces to molten metal, which is tapped at frequent intervals into torpedo-shaped rail wagons for onward transport to the steel furnace.
Limestone can be identified on aerial imagery by its signature light tone; here, a stock of lime is visible adjacent to the blast furnace, at lower left.
|
 |
|
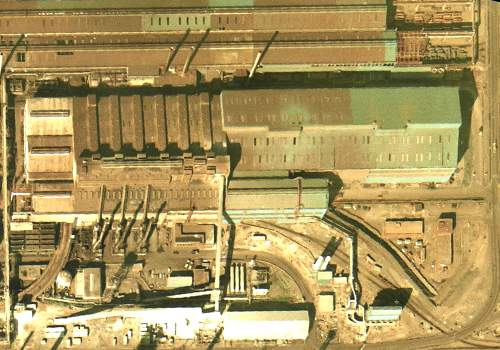
5. STEEL PRODUCTION
In the Basic Oxygen Steelmaking (BOS) plant seen here, high-pressure oxygen is blown into a vessel containing scrap metal and molten iron brought from the blast furnaces. The oxygen combines with unwanted elements such as carbon, leaving behind liquid steel. A flux of lime combines with the impurities to form slag, while carbon monoxide gas is collected for use elsewhere in the plant.
Many modern steel plants utilise a process of continuous casting (concast), whereby molten steel is cooled and shaped as it is poured from the furnace vessel. In this image, the concast building is visible to the right of the BOS plant, with a light-toned lime preparation building at photo south.
|
 |
|
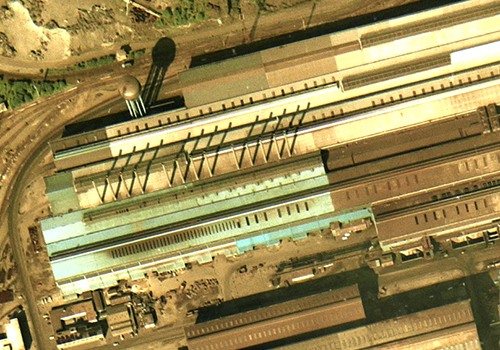
6. CASTING AND FINISHING
Rolling mills take the shaped steel from the concast building and form it into steel plates, coils and bars by re-heating, rolling, squeezing and carefully cooling the steel. It is then ready for further processing, such as cutting, coating and pressing, before it is used to create an end-product. Finished steel is then stockpiled outside the mill, ready for onward distribution. |
 |
|
7. POWER PLANT
A steelworks will normally have its own power station located within the site, to provide the large quantities of electricity needed around the plant. This is fuelled from the coal stockyard.
In this image, three Venturi-type cooling towers can be seen to the left of the power station; these cool hot water from the condensers. At the top of the image is the administration building, with staff car park adjacent.
|
 |